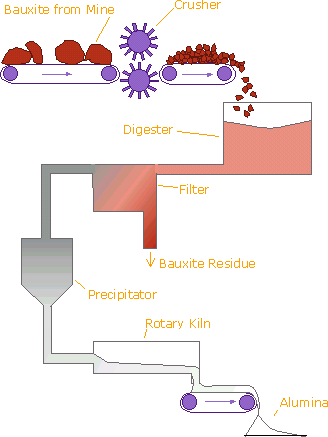
Bayer Process
- Bauxite, the most important ore of aluminum, contains only 30-54% alumina, Al2O3, the rest being a mixture of silica, various iron oxides, and titanium dioxide. The alumina must be purified before it can be refined to aluminum metal.
- In the Bayer process, bauxite is digested by washing with a hot solution of sodium hydroxide, NaOH, at 175°C. This converts the alumina to aluminum hydroxide, Al(OH)3, which dissolves in the hydroxide solution according to the chemical equation: Al2O3 + 2 OH- + 3 H2O → 2 [Al(OH)4]-
- The other components of bauxite do not dissolve. The solution is clarified by filtering off the solid impurities. The mixture of solid impurities is called red mud, and presents a disposal problem.